Медицинский активированный уголь
.webp)
Купить медицинский активированный уголь
Проблемы отрасли
Заблуждения в клинической практике
- Старые традиции (например, "1-часовое окно" для лечения отравлений) ограничивают применение некоторых мероприятий, хотя данные свидетельствуют в пользу более позднего введения (т.е. до 4 часов) некоторых токсинов.
- Риск прогрессирующей токсичности практически никогда не принимается во внимание, что приводит к различным стратегиям в чрезвычайных ситуациях.
Технические или производственные ограничения
- Контроль размера частиц имеет решающее значение для доставки лекарств (например, лимфатического таргетинга при лечении рака), а оптимизация наночастиц как по размеру, так и по площади поверхности может привести к агрегации и потере адсорбционной способности.
- Входящие в состав препарата вспомогательные вещества (например, диспергаторы) могут снизить эффективность загрузки.
Несоответствие нормативным требованиям и качеству
- Несколько фармакопей (таких как USP, EP, ChP) имеют различные специфические стандарты в отношении чистоты, адсорбции и предельного содержания эндотоксинов, что затрудняет соблюдение всех стандартов на международном уровне.
- Активированный уголь для инъекций" не имеет единой спецификации, что может повысить риск загрязнения альтернативных патологических препаратов.
Управление побочными эффектами
- Помимо перорального применения, запор является одной из задач лечения гиперфосфатемии, и на его долю приходятся вспомогательные средства, которые обычно включают в себя.
- Комбинации должны иметь определенную дозу, которая удовлетворяет двум требованиям: эффективность и переносимость желудочно-кишечного тракта.
различные виды активированного угля
-r8fslg51nt6wgjtvh6yldxb1gtkgm3lpe0oq1akgog.webp)
- Содержание йода: 600-1200
- Размер ячейки: 1×4/4×8/8×16/8×30/12×40/20×40/20×50/30×60/40×70 (другие размеры по запросу)
- Кажущаяся плотность: 400-700
-r8fsli0q1h9h3rr567ruiwtynlb71ht629zozuhoc0.webp)
- Содержание йода: 500-1300
- Размер ячейки: 0,9-1 мм/1,5-2 мм/3-4 мм/6 мм/8 мм (другие размеры по запросу)
- Кажущаяся плотность: 450-600
-r8fslbfupn0gui0p8mxgjghqhw7mjm31pdfamwrfjk.webp)
- Содержание йода: 500-1300
- Размер ячеек: 150/200/300/350 (другие размеры по запросу)
- Кажущаяся плотность: 450 - 550
-r8fsle9da54btbwls65c8xs4a1tq6pe8prdr2qn90w.webp)
- Содержание йода: 400-800
- Размер ячейки: 100×100×100 мм/100×100×50 мм (плотность ячеек по запросу)
- Кажущаяся плотность: 350-450
- Диаметр отверстия:1,5-8 мм

- Содержание йода: 700-1200 мг/г
- Площадь поверхности: 700-1200 м²/г
- Кажущаяся плотность: 320-550 кг/м³

- Содержание йода: 700-1200 мг/г
- Площадь поверхности: 700-1200 м²/г
- Кажущаяся плотность: 320-550 кг/м³

- Содержание йода: 700-1200 мг/г
- Площадь поверхности: 700-1200 м²/г
- Кажущаяся плотность: 300-650 кг/м³

- Содержание йода: 700-1200 мг/г
- Площадь поверхности: 700-1200 м²/г
- Кажущаяся плотность: 320-550 кг/м³

- Метод активации: Паровая/газовая активация при высоких температурах
- Структура пор: С преобладанием микропор, равномерное распределение пор
- Экологический профиль: Без химикатов, с низким содержанием золы
- Основные области применения: Газофазная адсорбция, очистка питьевой воды
- Метод активации: Химическая активация (например, H₃PO₄/ZnCl₂) при умеренных температурах
- Структура пор: Мезопористая, с высокой площадью поверхности
- Эффективность процесса: Сокращение времени активации, более высокий выход 30-50%
- Постобработка: Для удаления остатков требуется кислотная промывка

- Функционализация: Насыщенные активными агентами (например, I₂/Ag/KOH)
- Целевая адсорбция: Усиленное улавливание конкретных загрязняющих веществ (например, Hg⁰/H₂S/кислотных газов)
- Персонализация: Химическая оптимизация для целевых загрязнений
- Основные области применения: Очистка промышленных газов, защита от ХБРЯ
Почему стоит использовать наш активированный уголь

Профиль ультравысокой чистоты:
Соответствует строгим фармакопейным стандартам по содержанию тяжелых металлов и эндотоксинов, что очень важно для инъекционных и имплантируемых медицинских препаратов.
Точно рассчитанная пористость:
Оптимизированная структура пор обеспечивает превосходную кинетику адсорбции целевых токсинов и терапевтических агентов.

Согласованность между партиями:
Строгий контроль качества гарантирует надежную работу в протоколах экстренной и хронической терапии.

Поддержка нормативной документации:
Комплексные пакеты сертификации упрощают соблюдение глобальных требований к медицинским устройствам и фармацевтическим препаратам.

Готовность к модификации поверхности:
- Готовность к модификации поверхности: Настраиваемые функциональные группы обеспечивают расширенные возможности загрузки лекарств для целевой терапии.
Процесс и технология
1. Неотложная помощь при отравлениях
Обзор решений
Пероральный прием суспензии активированного угля для адсорбции проглоченных токсинов в желудочно-кишечном тракте перед системной абсорбцией.

Ключевые преимущества
- Адсорбция широкого спектра действия: Связывает различные токсины, включая фармацевтические препараты, пестициды и химикаты
- Быстрое действие: Механизм физической адсорбции действует в течение нескольких минут после введения
- Минимальная системная абсорбция: Остается в желудочно-кишечном тракте и выводится вместе со связанными токсинами
- Снижает заболеваемость: Ограничивает биодоступность токсинов и потенциал повреждения органов
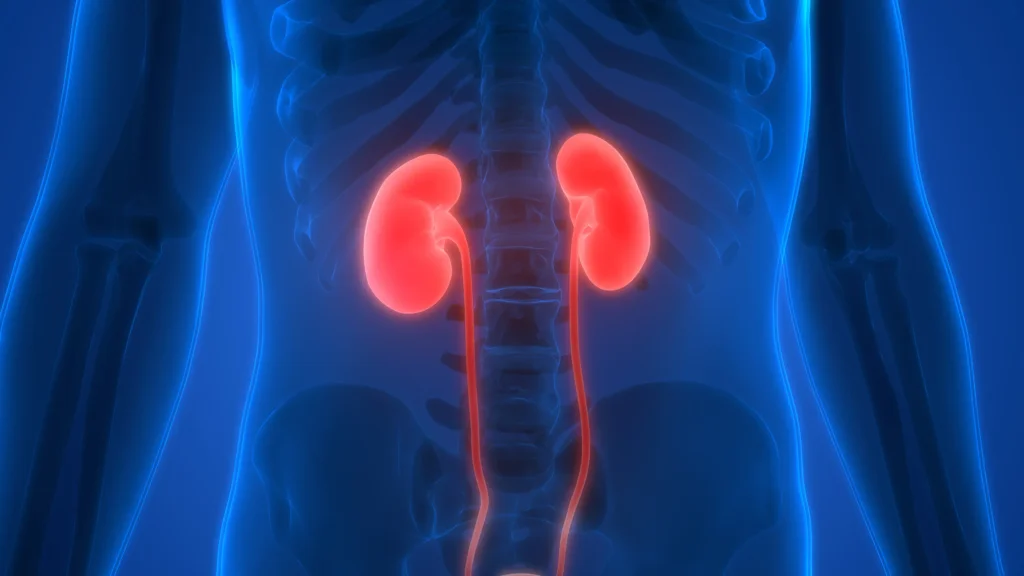
2. Управление хроническими заболеваниями почек
Обзор решений
Пероральные сорбенты на основе древесного угля, используемые в качестве фосфат-связывающих веществ для снижения всасывания фосфатов из рациона при гиперфосфатемии.
Ключевые преимущества
- Некальциевая альтернатива: Позволяет избежать риска кальцификации сосудов, связанного с традиционными вяжущими средствами
- Уменьшает минеральный дисбаланс: Специально нацелен на фосфатные ионы, сохраняя необходимые электролиты
- Действие ограничено кишечником: Действует местно без системных лекарственных взаимодействий
- Повышение комплаентности: Снижение нагрузки на таблетки по сравнению с некоторыми альтернативами
3. Местный уход за ранами
Обзор решений
Повязки с пропиткой из древесного угля для малоприятных, выделяющих воду ран, таких как грибковые опухоли или инфицированные язвы.

Ключевые преимущества
- Контроль запаха: Адсорбирует летучие соединения, вызывающие неприятный запах раны
- Связывание бактерий: Снижает микробную нагрузку на поверхность за счет физической адсорбции
- Устранение экссудата: Высокая способность впитывать жидкость поддерживает влажность раны
- Неадгезивные свойства: Минимизирует травмирование тканей при смене повязок
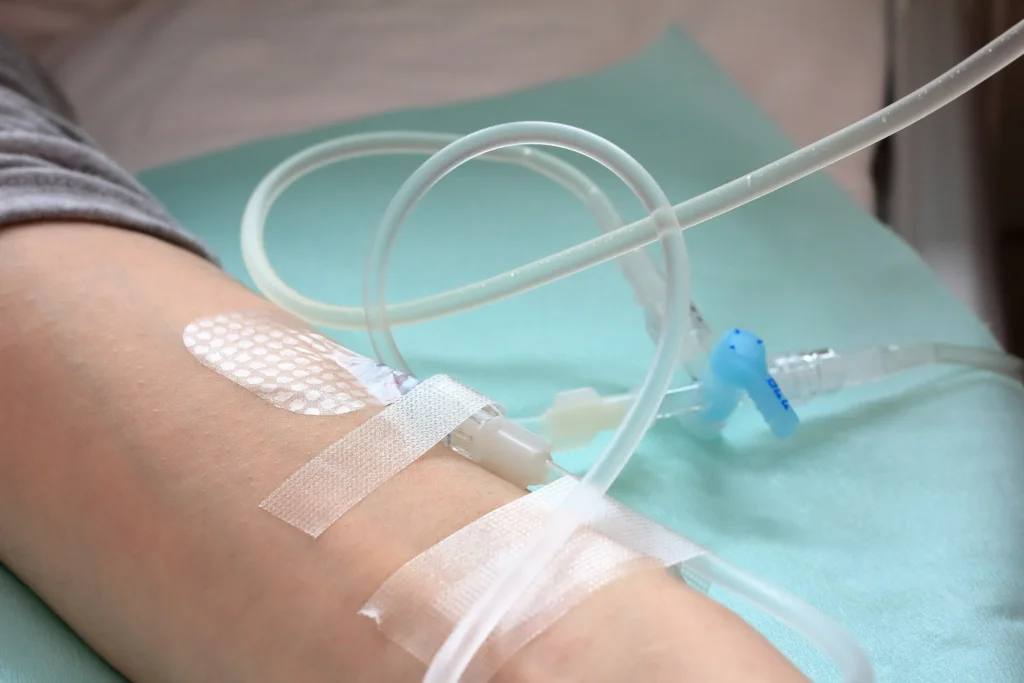
4. Улучшение доставки лекарств
Обзор решений
Угольные наночастицы как носители для адресной доставки терапевтических препаратов, особенно в онкологии.
Ключевые преимущества
- Контролируемое высвобождение: Изменяемая структура пор обеспечивает устойчивое выведение лекарств
- Лимфатическое таргетирование: Оптимизация размера частиц способствует накоплению в лимфатических узлах
- Защитная функция: Защищает лекарства от преждевременного разрушения в желудочно-кишечном тракте
- Синергетическая загрузка: Одновременная доставка нескольких терапевтических агентов

